Inside a Scientist's Day: What Really Slows the Work
A grounded look at the three recurring problems that shape every lab day—and the practical path to solving them.
A Three-Phase Journey
This page shows how a typical day in the lab can change over time – for scientists and for the teams that support them. We start from the reality today, show what Copilot and embedded AI can fix right now, then add proven third-party tools, and finally show the integrated state we aim for by 2027.
Low Effort
Copilot and built-in assistants
Medium Effort
Connected third-party AI tools
High Impact
Unified, intelligent R&D workspace
Three Persistent Challenges
These problems compound across data, operations, and compliance — making simple tasks take hours.

spent finding, cleaning, and re-analyzing data across ELN, LIMS, instruments, and spreadsheets.

caused by preventable issues like double bookings, expired calibrations, and stockouts.

time spent chasing missing fields, attachments, and signatures to make records inspection-ready.
What the Day Feels Like Today
Scientists and support teams see the same problems from different angles. These three snapshots reflect how the day actually feels.

Data: too many systems, not enough insight
A biologist starts the day trying to answer a simple question: "Did yesterday's assay work?" She opens the ELN, then plate reader software, then an old spreadsheet, then last month's analysis notebook. Ten tabs later, she still doesn't have a clean answer.

Operations: constant firefighting
A LabOps lead walks into double-booked instruments, a calibration that expired yesterday, and a MS Teams message asking why a critical reagent is out of stock again.

Compliance: fixing what should have been right
A QA scientist reviews a record flagged as incomplete—missing metadata, wrong SOP version, and three files sitting in email instead of the system. A routine study becomes a clean-up project.
What You Can Fix Now
These improvements use Copilot and AI assistants already available inside validated systems. They reduce friction immediately without changing core workflows.
Data: faster summaries and cleaner notes
- Summarize ELN entries and highlight missing information.
- Draft analysis notes from raw observations.
- Quickly answer, "What did we learn from this run?"
LabOps: hands-free capture at the bench
- Use voice to log steps and reagent usage.
- Set timers and reminders without leaving the bench.
- Capture more accurate run details with less typing.
Compliance: better records in less time
- Draft method descriptions and result summaries inside validated ELNs.
- Run quick checks for missing fields before routing for signature.
- Standardize language across similar studies.
Hands-free bench capture

AI drafting summary

How Scientists Could Use AI in Their Day-to-Day Work
Real, practical prompts that show how Copilot and AI assistants can help — starting now.
Data & Analysis
Summarize all runs in this experiment and highlight what changed.
Draft a results section based on these measurements.
Explain why plate 5 failed QC and suggest what to rerun.
Lab Operations
Log that I used 10 mL of buffer B in step 4.
Set a 10-minute timer for the incubation step.
Tell me if any instrument is showing drift today.
Compliance & Documentation
Check this record for missing metadata before I sign.
Show differences between batch 47 and batch 48 protocols.
Find all mass spec runs this month with purity <95%.
What You Can Fix in 3–9 Months
These changes require connecting instruments, ELN/LIMS, and quality systems through proven third-party platforms. They remove structural bottlenecks and benefit both scientists and support teams.
Data: harmonized, auto-QC'd, analysis-ready
- Instrument runs ingest automatically into the right project with complete metadata.
- QC rules run without manual steps and flag issues early.
- Historical datasets merge even when units or controls differ.

LabOps: predictive and coordinated
- Freezers and incubators send alerts before they drift, not after.
- Instruments rebook automatically when downtime is detected.
- Cross-site scheduling makes capacity and maintenance visible in one place.

Compliance: auto-attached, routed, complete
- Instrument outputs auto-attach to the correct record.
- Required fields are enforced at the moment of capture.
- Records route for signatures with clear SLAs and audit-ready trails.

Examples of enabling platforms include: data harmonization clouds, lab integration fabrics, LabOps telemetry, and enterprise QMS/ELN linkages.
North Star · End of 2026–2027
A unified, intelligent R&D workspace where data, operations, and compliance work together so scientists can focus on science.

Data: Continuous, contextualized data
Every run is captured, harmonized, QC'd, and linked to the right experiment automatically. Scientists ask questions like "Show me the pattern across all runs of this assay in the last 18 months," and get answers in minutes.
LabOps: Self-orchestrating operations
Instruments, inventory, and schedules coordinate automatically. Experiments start on time, methods are verified, and capacity is balanced across sites.
Compliance: Compliance in-flow
Records are complete, contemporaneous, and attributable as work happens. Submission packages assemble from the source system without manual stitching.
Why Not Jump Straight to 2027
Building the future requires a thoughtful, validated path
- Data needs to be harmonized before automation can make good decisions.
- Validated environments and change control matter more than speed.
- Scientists and support teams build trust through visible, incremental wins.
- Integration maturity varies across sites and systems; the path must respect that.
What Drives Adoption
Adoption is not about more features; it is about making everyday work meaningfully easier for both scientists and support teams.
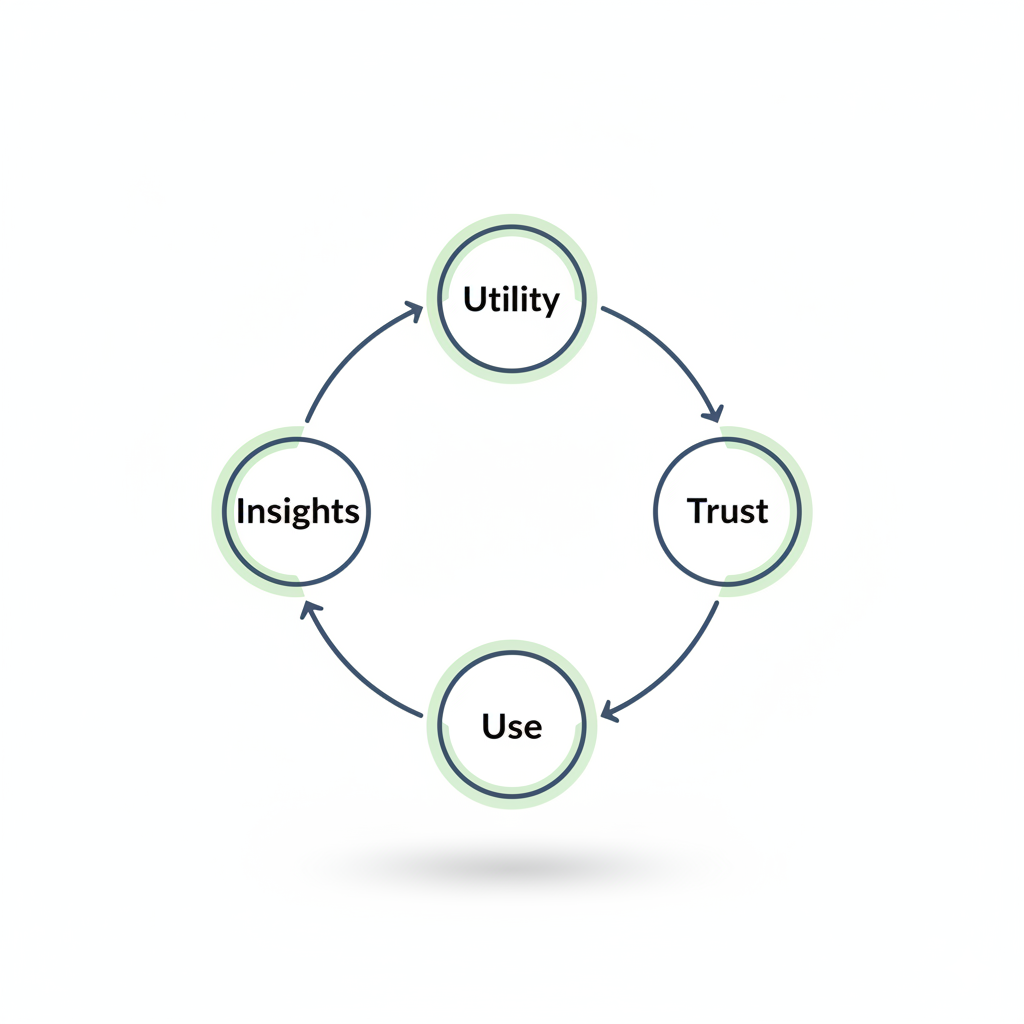
The adoption flywheel: utility → trust → use → insights → more utility
For scientists
- Help me find what I need quickly.
- Reduce repetitive steps and rework.
- Keep my data accurate without extra clicks.
For support teams
- Clear visibility into assets and processes.
- Fewer tickets for issues that automation can prevent.
- Better data for troubleshooting and audits.